
Chemistry, 18.02.2022 07:00 kordejah348
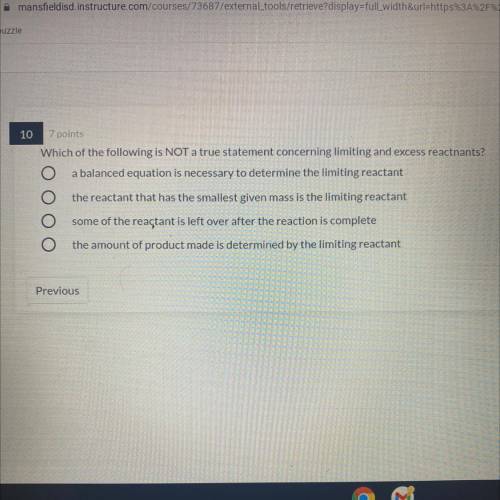
Which of the following is NOT a true statement concerning limiting and excess reactnants?
1. a balanced equation is necessary to determine the limiting reactant
2.the reactant that has the smallest given mass is the limiting reactant
3. some of the reactant is left over after the reaction is complete
4.the amount of product made is determined by the limiting reactant

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2



Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
Which of the following is NOT a true statement concerning limiting and excess reactnants?
1. a bal...
Questions



History, 04.10.2021 21:10


Mathematics, 04.10.2021 21:10

English, 04.10.2021 21:10


Mathematics, 04.10.2021 21:10

Mathematics, 04.10.2021 21:10





Social Studies, 04.10.2021 21:10


Mathematics, 04.10.2021 21:10

Mathematics, 04.10.2021 21:10

Mathematics, 04.10.2021 21:10




