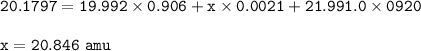Chemistry, 15.02.2022 03:40 jellyangie1
neon has 3 different isotopes, neon-20, neon-21, and neon-22. the first isotope has a mass 19.992 amu and an abundance of 90.60% . the second isotope (neon-21) has an abundance of 0.21%. the third isotope has a mass 21.991 amu and an abundance of 9.20%. what is the mass of the second isotope?

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 13:30
Explain the impact that changing the temperature has on a system in a state of dynamic equilibrium. what will happen when the temperature of an exothermic reaction mixture at equilibrium is increased?
Answers: 3

Chemistry, 23.06.2019 14:00
Which is not true regarding reaction rates? (2 points) catalysts are not used up in the reaction. catalysts speed up reactions by lowering the activation energy. reaction rates decrease as the concentration of reactants decrease. during reactions, concentrations of all reactants decrease at the same rate.
Answers: 1
You know the right answer?
neon has 3 different isotopes, neon-20, neon-21, and neon-22. the first isotope has a mass 19.992 am...
Questions





Mathematics, 14.01.2020 20:31



Chemistry, 14.01.2020 20:31

Mathematics, 14.01.2020 20:31

History, 14.01.2020 20:31


History, 14.01.2020 20:31

Advanced Placement (AP), 14.01.2020 20:31

Chemistry, 14.01.2020 20:31


Mathematics, 14.01.2020 20:31

Biology, 14.01.2020 20:31

Mathematics, 14.01.2020 20:31