
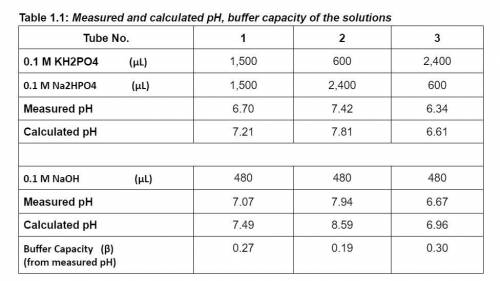
How does the ratio of weak acid and its conjugate base concentrations affect buffer capacity?
Which tube of solutions (molar concentrations of acid and base) is the best to maintain a near-constant pH?
What is the most effective pH range of the buffer based on its pKa value? Why?
How are different between the calculated and experimentally measured pH values of phosphate buffer solutions before and after adding 480 ul of 0.1 M NaOH solution?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
How does the ratio of weak acid and its conjugate base concentrations affect buffer capacity?
Whic...
Questions


Mathematics, 20.09.2020 14:01

History, 20.09.2020 14:01




Social Studies, 20.09.2020 14:01


Mathematics, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01


Biology, 20.09.2020 14:01


History, 20.09.2020 14:01

Geography, 20.09.2020 14:01



History, 20.09.2020 14:01




