
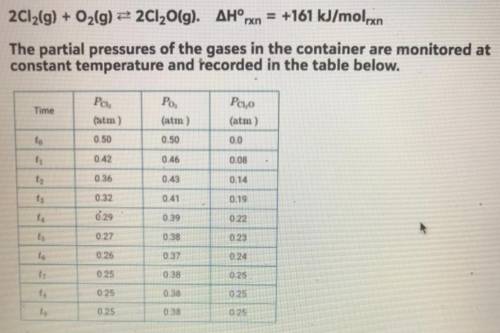
Equimolar amounts of Cl2 (g) and O2 (g) are injected into an evacuated, rigid container, where they react according to the equation below (see picture)
a. if 6.4 g of O2 (g) is consumed in the reaction with excess Cl2 (g), how many moles of Cl2O (g) are produced?
b. Which element is oxidized in this reaction? Justify your answer in terms of oxidation number.
c. At time t4, is the rate of the reverse reaction greater than, less than, or equal to the rate of the forward reaction? Justify your choice.
d. At equilibrium, the container holds fewer molecules of which gas, Cl2 (g) or O2 (g)? Explain your answer.
e. A student hypothesizes that if the temperature of the container is decreased after time t9, the mole fraction of Cl2O (g) in the container will increase. Do you agree or disagree with the student's hypothesis? Justify your answer.
f. Using the data in the table, determine the value of the equilibrium constant, Kp, for the reaction represented by the equation below.
2 Cl2 (g) + O2 (g) <-> 2 Cl2O (g)
g. Using the value determined in part (f), determine the value of Kp for the equation shown below.
4 Cl2 (g) + 2 O2 <-> 4 Cl2O (g)
h. Which gas, Cl2 (g) or O2 (g), will deviate most from the ideal gas law at low temperature? Justify your choice.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Equimolar amounts of Cl2 (g) and O2 (g) are injected into an evacuated, rigid container, where they...
Questions

Chemistry, 13.08.2020 17:01









Computers and Technology, 13.08.2020 17:01






History, 13.08.2020 17:01

Mathematics, 13.08.2020 17:01

Mathematics, 13.08.2020 17:01




