Chemistry, 13.02.2022 09:20 victoria8281
Determine the molar mass of (show your work) :
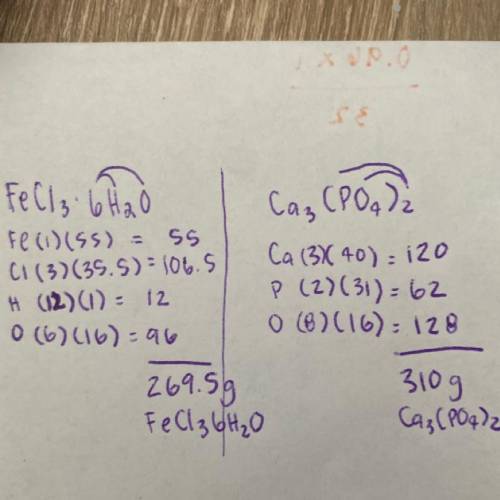
FeCl3•6H2O (note, this is all 1 molecule. Its an FeCl3 with a cage of 6H2O’s attached. These molecules are known as hydrates.)
Ca3(PO4)2

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Forests and meadows are often cut down to make way for farms or large number of new homes. what are some of the elements of ecosystems that are lost when plants in these areas are removed?
Answers: 2

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1
You know the right answer?
Determine the molar mass of (show your work) :
FeCl3•6H2O (note, this is all 1 molecule. Its an Fe...
Questions



Mathematics, 18.03.2021 01:30


Biology, 18.03.2021 01:30



Mathematics, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30



Mathematics, 18.03.2021 01:30

History, 18.03.2021 01:30

Chemistry, 18.03.2021 01:30

Arts, 18.03.2021 01:30




Mathematics, 18.03.2021 01:30

History, 18.03.2021 01:30