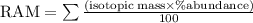Naturally occurring Indium has two isotopes.
4.28
%
4.28
%
of the atoms ar...

Chemistry, 13.02.2022 05:50 starfox5454
Naturally occurring Indium has two isotopes.
4.28
%
4.28
%
of the atoms are
Indium
−
113
Indium
-
113
(
113
In
113
In
) with a mass of
112.9
u
112.9
u
and
95.72
%
95.72
%
of the atoms are
Indium
−
115
Indium
-
115
(
115
In
115
In
) with a mass of
114.9
u
114.9
u
. Calculate the average atomic mass of Indium with the correct number of significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Questions

Physics, 13.09.2021 06:50


Mathematics, 13.09.2021 06:50





Mathematics, 13.09.2021 06:50

Physics, 13.09.2021 06:50


Mathematics, 13.09.2021 06:50

Mathematics, 13.09.2021 06:50

History, 13.09.2021 06:50




English, 13.09.2021 06:50

History, 13.09.2021 06:50


Chemistry, 13.09.2021 06:50