
Chemistry, 13.02.2022 04:40 DesireeGuzman5213
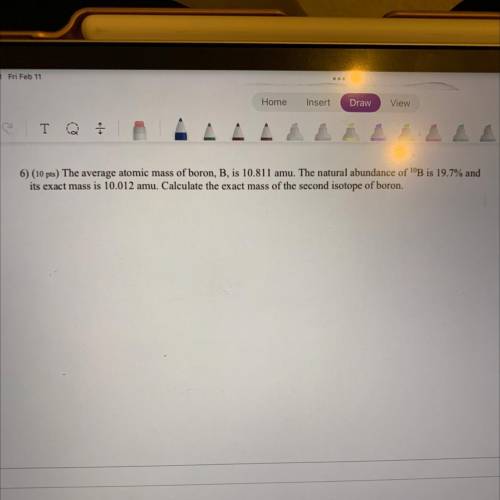
6) The average atomic mass of boron, B, is 10.811 amu. The natural abundance of 10B is 19.7% and it’s exact mass is 10.012 amu. Calculate the exact mass of the second isotope of boron.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1


Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1
You know the right answer?
6) The average atomic mass of boron, B, is 10.811 amu. The natural abundance of 10B is 19.7% and it’...
Questions

Physics, 15.04.2020 20:07

Health, 15.04.2020 20:07





Mathematics, 15.04.2020 20:07

Biology, 15.04.2020 20:07







History, 15.04.2020 20:07


History, 15.04.2020 20:07

Mathematics, 15.04.2020 20:07




