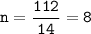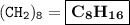Chemistry, 08.02.2022 14:00 tatilynnsoto17
A compound with the empirical formula CH2 was found to have a molar mass of approximately 112 g. Write the molecular formula of the compound.
2Points
Show all your work. Please use correct formatting for subscripts and exponents. The math formula editor makes it easier to show work.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
A compound with the empirical formula CH2 was found to have a molar mass of approximately 112 g. Wri...
Questions

English, 02.09.2021 20:10


Business, 02.09.2021 20:10

Business, 02.09.2021 20:10



Mathematics, 02.09.2021 20:20

Mathematics, 02.09.2021 20:20


Mathematics, 02.09.2021 20:20




Computers and Technology, 02.09.2021 20:20


Mathematics, 02.09.2021 20:20

Biology, 02.09.2021 20:20



Mathematics, 02.09.2021 20:20