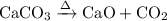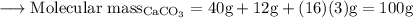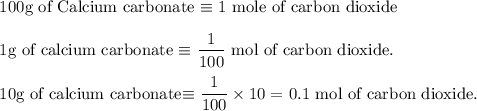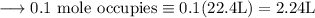Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1
You know the right answer?
Write an equation for the thermal decomposition of CaCO3. Determine the volume of CO2 measured at s...
Questions

Mathematics, 13.02.2021 01:00


Mathematics, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00


Mathematics, 13.02.2021 01:00


Chemistry, 13.02.2021 01:00

SAT, 13.02.2021 01:00

Chemistry, 13.02.2021 01:00


Mathematics, 13.02.2021 01:00


Mathematics, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00

Mathematics, 13.02.2021 01:00



Arts, 13.02.2021 01:00