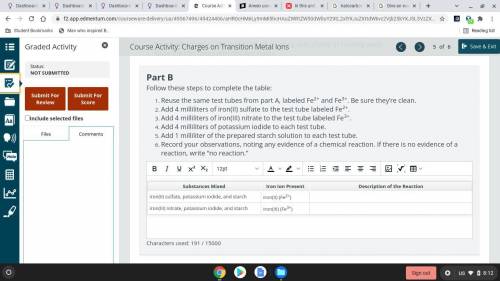
Part B
Follow these steps to complete the table:
Reuse the same test tubes from part A...

Chemistry, 03.02.2022 08:50 angelteddy033
Part B
Follow these steps to complete the table:
Reuse the same test tubes from part A, labeled Fe2+ and Fe3+. Be sure they’re clean.
Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2+.
Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe3+.
Add 4 milliliters of potassium iodide to each test tube.
Add 1 milliliter of the prepared starch solution to each test tube.
Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a reaction, write “no reaction.”

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Questions





Mathematics, 25.03.2020 16:54


Mathematics, 25.03.2020 16:54



Computers and Technology, 25.03.2020 16:54






Biology, 25.03.2020 16:55

English, 25.03.2020 16:55



Mathematics, 25.03.2020 16:56



