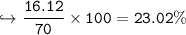Chemistry, 01.02.2022 21:50 annamerryberry1016
In this reaction: Mg (s) + I₂ (s) → MgI₂ (s), if 10.0 g of Mg reacts with 60.0 g of I₂, and 53.88 g of MgI₂ form, what is the percent yield?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
In this reaction: Mg (s) + I₂ (s) → MgI₂ (s), if 10.0 g of Mg reacts with 60.0 g of I₂, and 53.88 g...
Questions

Mathematics, 08.06.2021 18:10

Mathematics, 08.06.2021 18:10

Chemistry, 08.06.2021 18:10


Physics, 08.06.2021 18:10






Mathematics, 08.06.2021 18:10

Mathematics, 08.06.2021 18:10






Business, 08.06.2021 18:10