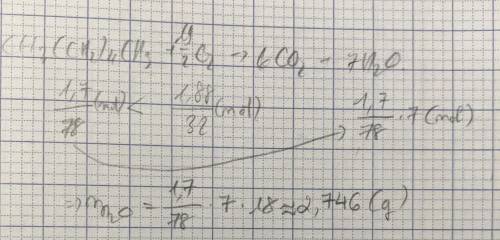Chemistry, 01.02.2022 16:50 surfergirlmymy
Suppose 1.7 g of hexane is mixed with 1.88 g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3


You know the right answer?
Suppose 1.7 g of hexane is mixed with 1.88 g of oxygen. Calculate the maximum mass of water that cou...
Questions


English, 05.04.2020 00:42



Chemistry, 05.04.2020 00:42




Mathematics, 05.04.2020 00:43

Arts, 05.04.2020 00:43



Mathematics, 05.04.2020 00:43



Mathematics, 05.04.2020 00:43

Chemistry, 05.04.2020 00:43



History, 05.04.2020 00:43