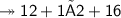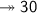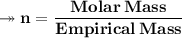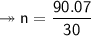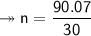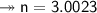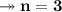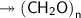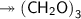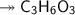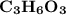Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 23:40
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2

Chemistry, 23.06.2019 04:31
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3

Chemistry, 23.06.2019 15:40
Twenty-seven milliliters of an acid with an unknown concentration are titrated with a base that has a concentration of 0.55 m. the indicator changed color when 12.5 milliliters of base were added. what is the concentration of the unknown acid?
Answers: 2
You know the right answer?
Molar mass of a compound is 90.07 g/mol. Determine its molecular formula if its empirical
formula...
Questions



English, 22.09.2021 19:10


Mathematics, 22.09.2021 19:10

English, 22.09.2021 19:10

Mathematics, 22.09.2021 19:10


Biology, 22.09.2021 19:10

Health, 22.09.2021 19:10


World Languages, 22.09.2021 19:10


Mathematics, 22.09.2021 19:10

Business, 22.09.2021 19:10


Advanced Placement (AP), 22.09.2021 19:20

Physics, 22.09.2021 19:20

Biology, 22.09.2021 19:20

 ☀️
☀️
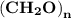
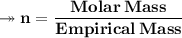
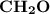 –
–