
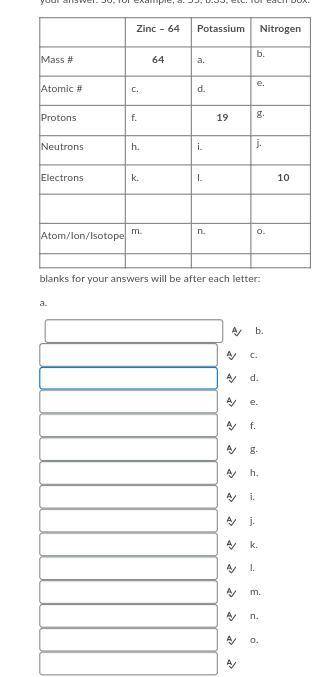
Question 25 options:
Complete the following table by listing your answers a - o in the blanks below.
To make sure you get the correct blank to your answer, please put the letter with your answer. So, for example, a. 55, b.33, etc. for each box.
blanks for your answers will be after each letter:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 08:30
Since the gas in your graduated cylinder is a mixture of butane and water vapor, you must determine the partial pressure of the butane, pbutane, alone. to do this, consult a reference and record the partial pressure of the water vapor, pwater, at the temperature you recorded. use the following formula to compute the partial pressure of the butane. pbutane = atmosphere - pwater use the following combined gas law formula and compute the volume that the butane sample will occupy at stp. (hint: convert both temperatures to kelvin.) pbutane x voriginal = pstandard x vfinal troom tstandard use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. grams of butane you used “x” grams of butane ml of butane corrected to stp = 22,400 ml compute the theoretical molar mass of butane based on its formula and the atomic masses on the periodic table. compare your experimental results from #3 to the theoretical value of #4, computing a percent error of your findings using this formula: % error = measured value - accepted value x 100 accepted value use the following ratio and proportion formula to determine the mass of butane needed to occupy a volume of 22.4 l at stp. need asap
Answers: 1

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
Question 25 options:
Complete the following table by listing your answers a - o in the blanks bel...
Questions



Mathematics, 21.07.2019 03:20


Mathematics, 21.07.2019 03:20

Health, 21.07.2019 03:20

Computers and Technology, 21.07.2019 03:20





English, 21.07.2019 03:20

History, 21.07.2019 03:20

Mathematics, 21.07.2019 03:20

History, 21.07.2019 03:20







