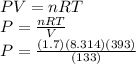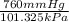Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
If I contain 1.7 moles of gas in a container with a volume of 133 liters and at a temperature of 393...
Questions

Mathematics, 02.04.2020 00:53

Biology, 02.04.2020 00:53


English, 02.04.2020 00:53









SAT, 02.04.2020 00:53

Mathematics, 02.04.2020 00:53

Mathematics, 02.04.2020 00:53


Mathematics, 02.04.2020 00:53