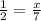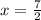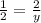Chemistry, 24.01.2022 05:10 jacquelineS4232
In this equation 2 K + 1 MgBr2 → 2 KBr + 1 Mg. If you produce 7 mols of KBr, how many mols of MgBr2 did you need? If I have 2 moles of MgBr2, how much KBr can I make in moles?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1
You know the right answer?
In this equation 2 K + 1 MgBr2 → 2 KBr + 1 Mg. If you produce 7 mols of KBr, how many mols of MgBr2...
Questions


History, 31.05.2021 03:00

Chemistry, 31.05.2021 03:00

Mathematics, 31.05.2021 03:00

Chemistry, 31.05.2021 03:00

Mathematics, 31.05.2021 03:00

History, 31.05.2021 03:00


History, 31.05.2021 03:00



Mathematics, 31.05.2021 03:00