HELP PLEASE
Use the following steps to balance the redox reaction below:
Mg + Au+ Mg2+ + Au<...

Chemistry, 23.01.2022 14:00 sarahgrindstaff123
HELP PLEASE
Use the following steps to balance the redox reaction below:
Mg + Au+ Mg2+ + Au
a. Write the oxidation and reduction half-reactions. Make sure each half-reaction is balanced for number of atoms
and charge. (3 points)
b. Multiply each half reaction by the correct number in order to balance charges for the two half reactions
c. Add the equations and simplify to get a balanced equation
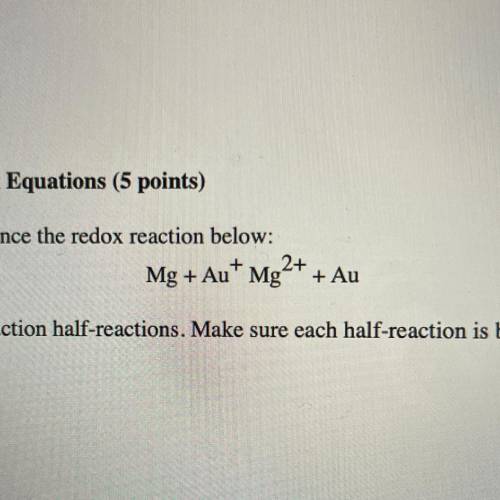

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1
You know the right answer?
Questions



Advanced Placement (AP), 11.01.2020 11:31




Social Studies, 11.01.2020 11:31

Mathematics, 11.01.2020 11:31

Social Studies, 11.01.2020 11:31

Social Studies, 11.01.2020 11:31

Biology, 11.01.2020 11:31

History, 11.01.2020 11:31






Mathematics, 11.01.2020 11:31

Biology, 11.01.2020 11:31



