
Chemistry, 21.01.2022 14:00 wirchakethan23
If 3.50 mol of ethane, CzH6, undergo combustion according to the unbalaced equation given of oxygen requires ? How many moles of each product formed?
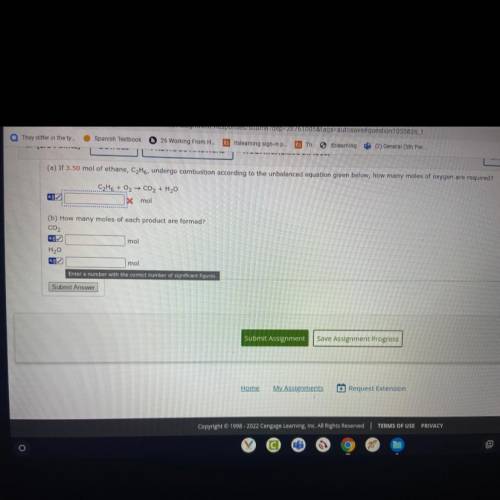

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 19:20
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
If 3.50 mol of ethane, CzH6, undergo combustion according to the unbalaced equation given of oxygen...
Questions








Computers and Technology, 01.11.2019 01:31









Mathematics, 01.11.2019 01:31



Social Studies, 01.11.2019 01:31



