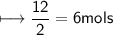Chemistry, 21.01.2022 14:00 melvina13bday
You are asked to make 12 moles of iron(Fe) from iron oxide and carbon monoxide.
Fe2O3(s) + 3CO(g)→2Fe(l) + 3CO2(g)
Approximately how many moles of iron oxide(Fe2O3) is used?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

You know the right answer?
You are asked to make 12 moles of iron(Fe) from iron oxide and carbon monoxide.
Fe2O3(s) + 3CO(g)→...
Questions






Advanced Placement (AP), 29.04.2021 22:30


Mathematics, 29.04.2021 22:30

Mathematics, 29.04.2021 22:30



Chemistry, 29.04.2021 22:30

Mathematics, 29.04.2021 22:30

Mathematics, 29.04.2021 22:30



Mathematics, 29.04.2021 22:30