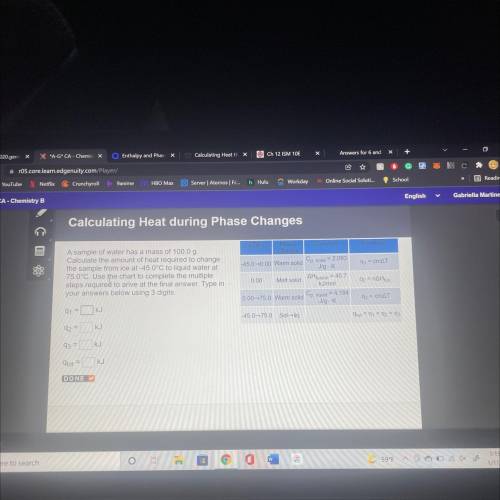
A sample of water has a mass of 100.00
Calculate the amount of heat required to change
the s...

Chemistry, 19.01.2022 14:00 somethingar183
A sample of water has a mass of 100.00
Calculate the amount of heat required to change
the sample from ice at 450°C to liquid water at
78.0°C. Use the chart to complete the multiple
steps required to arrive at the final answer. Type in your answers below using 3 digits.
Q1= KJ
Q2= KJ
Q3= KJ
q(tot)= KJ

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3
You know the right answer?
Questions

Mathematics, 12.04.2020 23:17

English, 12.04.2020 23:17



Mathematics, 12.04.2020 23:17




History, 12.04.2020 23:17




Mathematics, 12.04.2020 23:17


Health, 12.04.2020 23:17





History, 12.04.2020 23:17



