
Chemistry, 18.01.2022 03:10 anferneebcoleman
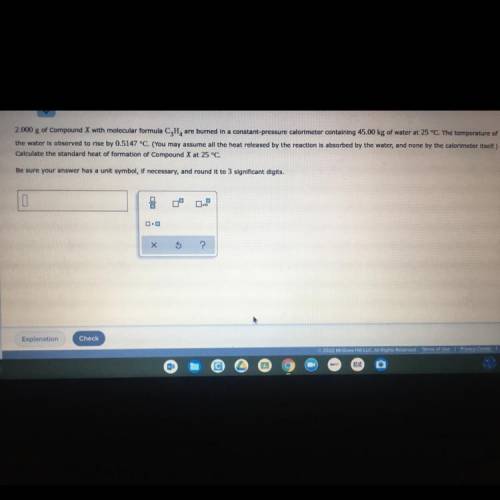
2.000g of compound X with molecular formula C3H4 are burned into a constant pressure calorimeter containing 45.00 kg of water at 25 degrees Celsius. The temperature of water is observed to rise by 0.5147 degrees Celsius (you may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) calculate the standard heat of formation of compound X at 25 degree Celsius.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2


Chemistry, 23.06.2019 03:30
Name atleast 3 type of energy associated with the microwave
Answers: 1
You know the right answer?
2.000g of compound X with molecular formula C3H4 are burned into a constant pressure calorimeter con...
Questions






















