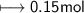Chemistry, 16.01.2022 05:50 ashleyvalles16
Calculate the number of moles of SO2 required to produce 17 g of CO in the following chemical equation: 5 C + 2 SO2 → CS2 + 4 CO

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1
You know the right answer?
Calculate the number of moles of SO2 required to produce 17 g of CO in the following chemical equati...
Questions


Mathematics, 10.11.2020 14:00



Health, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00




Mathematics, 10.11.2020 14:00

Mathematics, 10.11.2020 14:00




Mathematics, 10.11.2020 14:00


Computers and Technology, 10.11.2020 14:00


Mathematics, 10.11.2020 14:00

French, 10.11.2020 14:00