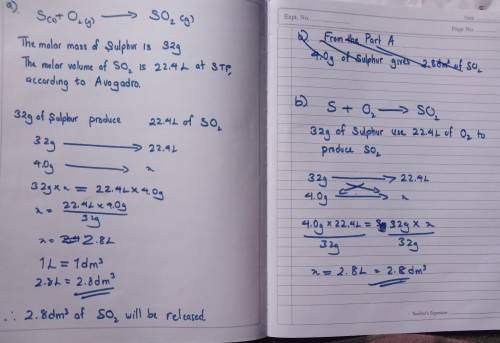Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
You know the right answer?
exactly 4.0g of sulfur is burnt in a fume extraction cupboard. The sulfur is ignited in an excess of...
Questions

History, 12.03.2021 22:30




History, 12.03.2021 22:30


Mathematics, 12.03.2021 22:30

Mathematics, 12.03.2021 22:30



Mathematics, 12.03.2021 22:30



Mathematics, 12.03.2021 22:30



Computers and Technology, 12.03.2021 22:30


English, 12.03.2021 22:30