
Chemistry, 06.01.2022 02:30 priceisright11401
PLEASE HELP ASAP WILL GIVE BRAINLIEST
(dont give chegg link pls)
How much heat is required to convert 150 g of liquid H2O at 43.5 °C into steam at 130 °C?
Specific heat of liquid water is 4.18 J/g oC.
Specific heat of water vapor is 1.84 J/g oC.
ΔHvap = 40.67 kJ/mol

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
PLEASE HELP ASAP WILL GIVE BRAINLIEST
(dont give chegg link pls)
How much heat is req...
How much heat is req...
Questions




Mathematics, 31.03.2020 05:41

Mathematics, 31.03.2020 05:41

Mathematics, 31.03.2020 05:41

Mathematics, 31.03.2020 05:41

Social Studies, 31.03.2020 05:41





Mathematics, 31.03.2020 05:42

Biology, 31.03.2020 05:42


Geography, 31.03.2020 05:42


Social Studies, 31.03.2020 05:42


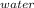 *ΔT
*ΔT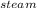 * ΔT
* ΔT

