
Chemistry, 05.01.2022 19:30 natalie857123
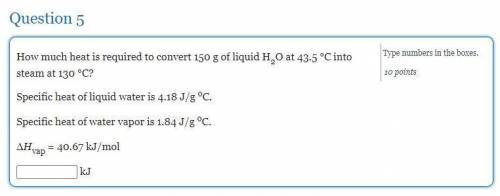
How much heat is required to convert 150 g of liquid H2O at 43.5 °C into steam at 130 °C?
Specific heat of liquid water is 4.18 J/g oC.
Specific heat of water vapor is 1.84 J/g oC.
ΔHvap = 40.67 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
How much heat is required to convert 150 g of liquid H2O at 43.5 °C into steam at 130 °C?
Specific...
Questions



Mathematics, 06.10.2020 14:01

History, 06.10.2020 14:01



History, 06.10.2020 14:01


Chemistry, 06.10.2020 14:01





Mathematics, 06.10.2020 14:01






Mathematics, 06.10.2020 14:01



