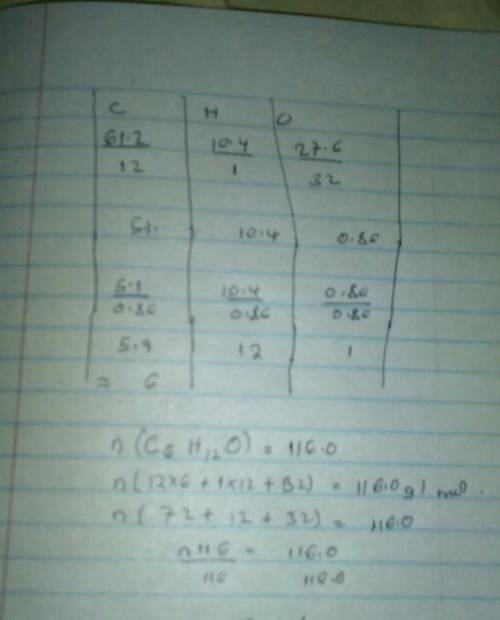Chemistry, 01.01.2022 19:50 geminigirl077
#4: Determine the molecular formula of o compound that is made up of 61.2 g carbon, 10.4 g hydrogen and 27.6 g oxygen if its molar mass is 116.0 g/mole. I will give Brainliest to best answer + SHOWS WORK!! fake answers will be reported and deleted. <3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
#4: Determine the molecular formula of o compound that is made up of 61.2 g carbon, 10.4 g hydrogen...
Questions

Mathematics, 23.12.2020 19:00


Computers and Technology, 23.12.2020 19:00

English, 23.12.2020 19:00

Mathematics, 23.12.2020 19:00

Mathematics, 23.12.2020 19:00



Chemistry, 23.12.2020 19:00

Mathematics, 23.12.2020 19:00



English, 23.12.2020 19:00


Mathematics, 23.12.2020 19:10

Mathematics, 23.12.2020 19:10



Mathematics, 23.12.2020 19:10