
PLZ HELP Stoichiometry Calculation
Imagine that you are solving the following stoichiometry problem:
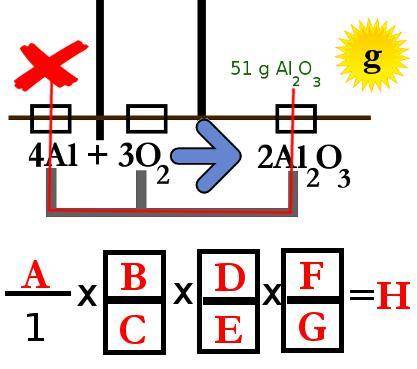
When aluminum oxidizes in air, it forms aluminum oxide (Al2O3):
4Al + 3O2 --> 2Al2O3 if a 51g sheet of aluminum oxide formed completely in excess oxygen, how many grams of aluminum were oxidized? Using the magic mole method, you diagram the problem, map out your solution route, and convert your diagram to an equation.
Use this diagram to answer the problems below.
(diagram included
2.What units go in spot C in the equation above?
mol Al2O3. mol. g. g Al2O3
3.What units go in spot B in the equation above?
g Al. mol Al2O3. mol Al. g Al2O3
4.What number goes in spot D in the equation? Enter the number only without any units
5.What number goes in spot E in the equation above? (Enter the number only

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
PLZ HELP Stoichiometry Calculation
Imagine that you are solving the following stoichiometry proble...
Questions

Chemistry, 05.01.2021 18:00




Mathematics, 05.01.2021 18:00



History, 05.01.2021 18:00

History, 05.01.2021 18:00


Chemistry, 05.01.2021 18:00

World Languages, 05.01.2021 18:00


Mathematics, 05.01.2021 18:00



Mathematics, 05.01.2021 18:00

Mathematics, 05.01.2021 18:00


Mathematics, 05.01.2021 18:00



