
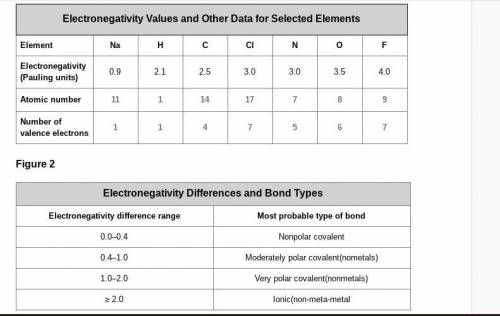
A cesium (Cs) atom has an atomic number of 55 and one electron in its outer shell. Considering the data in Figure 1 and Figure 2, what electronegativity value would you expect it to have, and what kind of bond is it likely to form with a chlorine atom? Explain your reasoning.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


You know the right answer?
A cesium (Cs) atom has an atomic number of 55 and one electron in its outer shell. Considering the d...
Questions

Mathematics, 25.12.2021 19:00


Mathematics, 25.12.2021 19:00

Social Studies, 25.12.2021 19:00


Biology, 25.12.2021 19:00

Mathematics, 25.12.2021 19:00

Chemistry, 25.12.2021 19:00



Mathematics, 25.12.2021 19:10

Physics, 25.12.2021 19:10


English, 25.12.2021 19:10

Mathematics, 25.12.2021 19:10


Mathematics, 25.12.2021 19:10


Social Studies, 25.12.2021 19:10

Mathematics, 25.12.2021 19:10



