What is the mass of 8.65 x 10^23 molecules of N2Cl4
A. 1.437 g
B. 244.019 g
C. 0.696 g...

Chemistry, 14.12.2021 21:50 baeethtsadia
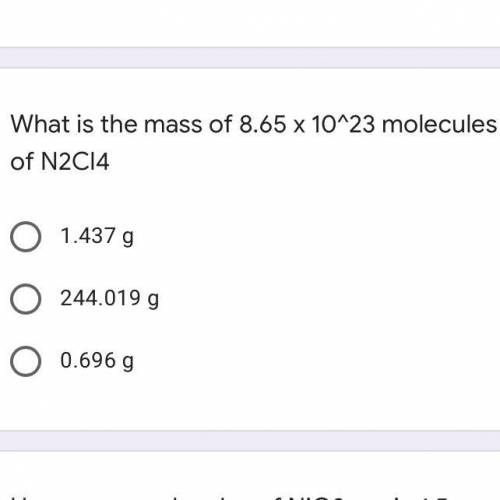
What is the mass of 8.65 x 10^23 molecules of N2Cl4
A. 1.437 g
B. 244.019 g
C. 0.696 g

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2
You know the right answer?
Questions

Mathematics, 09.10.2019 12:10


Social Studies, 09.10.2019 12:10




Mathematics, 09.10.2019 12:10


Health, 09.10.2019 12:10

Biology, 09.10.2019 12:10

Mathematics, 09.10.2019 12:10

Mathematics, 09.10.2019 12:10



Mathematics, 09.10.2019 12:10

Mathematics, 09.10.2019 12:10

English, 09.10.2019 12:10






