Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1


Chemistry, 23.06.2019 03:00
Air pressure is measured in pascals. for a professional american football game, the ball should be inflated to about 90,000 pascals. scientists studied the effects of air temperature on the pressure inside american footballs by taking these steps: 1. prepare 100 footballs. 2. measure each football's air pressure. 3. divide footballs into 10 groups. 4. place the groups in different lockers cooled to different air temperatures. 5. after 12 hours, remove the footballs from lockers. 6. measure each football's pressure again. 7. compare the new pressures to the starting pressures. what two terms best describe the variable "air pressure inside the football" in this experiment? independent, qualitative independent, quantitative dependent, qualitative dependent, quantitative
Answers: 3

You know the right answer?
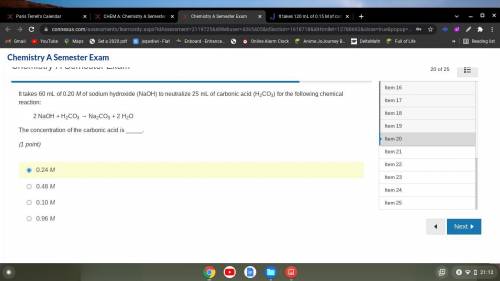
It takes 60 mL of 0.20 M of sodium hydroxide (NaOH) to neutralize 25 mL of carbonic acid (H2CO3) for...
Questions

Mathematics, 30.01.2021 06:10

Arts, 30.01.2021 06:10

English, 30.01.2021 06:10

Mathematics, 30.01.2021 06:10


English, 30.01.2021 06:10

English, 30.01.2021 06:10

Mathematics, 30.01.2021 06:10

Mathematics, 30.01.2021 06:10

Mathematics, 30.01.2021 06:10

Computers and Technology, 30.01.2021 06:10

Mathematics, 30.01.2021 06:10



Chemistry, 30.01.2021 06:10


English, 30.01.2021 06:10



Chemistry, 30.01.2021 06:10