Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
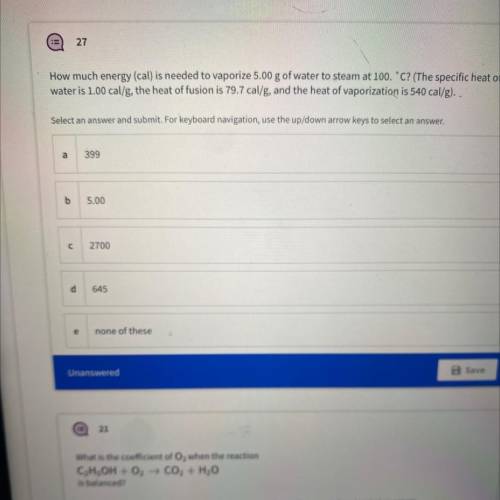
How much energy (cal) is needed to vaporize 5.00 g of water to steam at 100.C? (The specific heat of...
Questions






Mathematics, 21.04.2021 09:40

Mathematics, 21.04.2021 09:40

Mathematics, 21.04.2021 09:40



Mathematics, 21.04.2021 09:40

Chemistry, 21.04.2021 09:40


History, 21.04.2021 09:40


Mathematics, 21.04.2021 09:40

Mathematics, 21.04.2021 09:40



Advanced Placement (AP), 21.04.2021 09:40