
Chemistry, 13.12.2021 06:00 ultimatesaiyan
25.0g of iron is heated to 100.0 and then placed in 50.0 g of water in a insulated calorimeter. the initial temperature of the water is 38.00. the specific heat of water is 4.181j/g and the specific heat if iron is 0.45j/g. what is the final temp of the water and the iron?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1

You know the right answer?
25.0g of iron is heated to 100.0 and then placed in 50.0 g of water in a insulated calorimeter. the...
Questions

Mathematics, 01.06.2020 20:57





Mathematics, 01.06.2020 20:57


Mathematics, 01.06.2020 20:57

History, 01.06.2020 20:57

Chemistry, 01.06.2020 20:57


Mathematics, 01.06.2020 20:57

Mathematics, 01.06.2020 20:57

Mathematics, 01.06.2020 20:57

Engineering, 01.06.2020 20:57



History, 01.06.2020 20:57

Mathematics, 01.06.2020 20:57

Mathematics, 01.06.2020 20:57

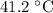 .
.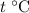 be the final temperature of the water and the iron.
be the final temperature of the water and the iron.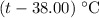 .
.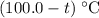 .
. denote the specific heat of each material. Let
denote the specific heat of each material. Let 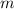 denote the mass of the material. For a temperature change of
denote the mass of the material. For a temperature change of 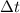 , the energy change involved would be:
, the energy change involved would be: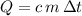 .
.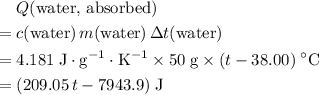 .
.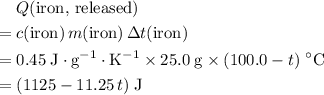 .
.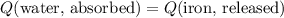 .
.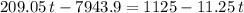 .
.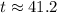 .
.

