
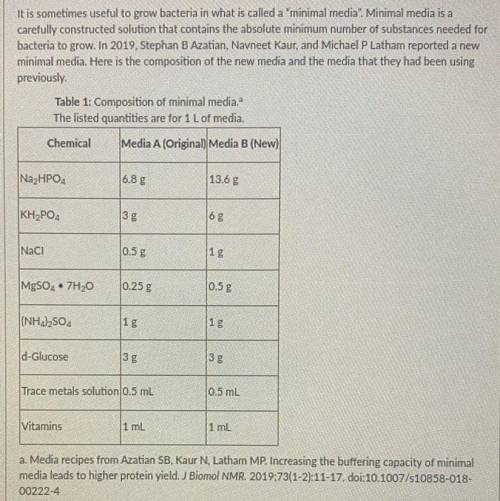
Q1. Which two chemicals in the table above are most likely act together as a buffer? Support your claim using what you know about buffers and how they work.
Q2. What is the difference between the old media and the new media? What impact does this have on the ability of the new media to buffer changes in pH?
Q3. As bacteria grow, they produce acidic metabolic waste products that gradually slow down the rate of cell division. Based on this, which media would you expect to allow the bacteria to grow for a longer period of time before the pH goes up rapidly and cell growth slows down? Include the reasons supporting your prediction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3


Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
Q1. Which two chemicals in the table above are most likely act together as a buffer? Support your cl...
Questions


Physics, 28.01.2021 21:00



Mathematics, 28.01.2021 21:00

Mathematics, 28.01.2021 21:00

Chemistry, 28.01.2021 21:00


English, 28.01.2021 21:00


Mathematics, 28.01.2021 21:00


Mathematics, 28.01.2021 21:00

Biology, 28.01.2021 21:00

Mathematics, 28.01.2021 21:00

Mathematics, 28.01.2021 21:00

Mathematics, 28.01.2021 21:00

Biology, 28.01.2021 21:00

Arts, 28.01.2021 21:00

Mathematics, 28.01.2021 21:00



