
Chemistry, 10.12.2021 04:10 mylittleponeyfbrarit
Prediction: The Cucl, crystal will dissolve in water. If we write the solvation process as a chemical
equation, we can predict two possible outcomes:
a. Cuci
H2O Cu?* (aq) + 2 Cl(aq)
H2O Cu2+ (aq) + Cl2(9)
+ 9
.
b. Cuciz
In equation (a) Cl' ions remain dissolved in water whereas in equation (b) the chlorine molecule CI,
forms and leaves the solution as a gas. Which outcome do you expect (a or b)? Explain your reasoning.
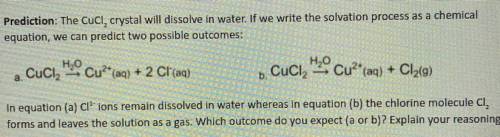

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
Prediction: The Cucl, crystal will dissolve in water. If we write the solvation process as a chemica...
Questions

Mathematics, 12.10.2019 02:50


Biology, 12.10.2019 02:50


Biology, 12.10.2019 02:50




Biology, 12.10.2019 02:50

Mathematics, 12.10.2019 02:50


Mathematics, 12.10.2019 02:50




Mathematics, 12.10.2019 02:50

History, 12.10.2019 02:50






