
Chemistry, 09.12.2021 08:40 Savannahpeeler1001
[Review Toples)
In radical chlorination of alkanes, non-equivalent hydrogens react with chlorine atoms at different rates. At 35 °C, primary, secondary, and tertiary C-H bonds
react at relative rates of 1:3.9: 5.2 respectively.
These are conditions of kinetic control where product ratios are determined by relative rates of formation. For example, if A is formed twice as fast as B, the A:B
product ratio will be 2.
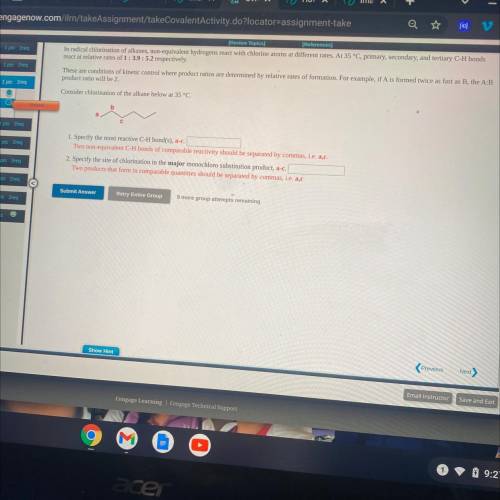
Consider chlorination of the alkane below at 35 °C.
1. Specify the most reactive C-H bond(s), a-c.
Two non-equivalent C-H bonds of comparable reactivity should be separated by commas, i. e. a, c.
2. Specify the site of chlorination in the major monochldro substitution product, a-c.
Two products that form in comparable quantities should be separated by commas, i. e. a, c

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 09:00
The vapor pressure of water at 25.0°c is 23.8 torr. determine the mass of glucose (molar mass = 180 g/mol) needed to add to 500.0 g of water to change the vapor pressure to 22.8 torr.
Answers: 1
You know the right answer?
[Review Toples)
In radical chlorination of alkanes, non-equivalent hydrogens react with chlorine a...
Questions

Mathematics, 30.03.2021 04:50

English, 30.03.2021 04:50


Physics, 30.03.2021 04:50

English, 30.03.2021 04:50

Social Studies, 30.03.2021 04:50

World Languages, 30.03.2021 04:50

History, 30.03.2021 04:50

Computers and Technology, 30.03.2021 04:50

SAT, 30.03.2021 05:00


Mathematics, 30.03.2021 05:00



Mathematics, 30.03.2021 05:00


English, 30.03.2021 05:00

Mathematics, 30.03.2021 05:00

Mathematics, 30.03.2021 05:00



