A substance (A) reacts to form another substance (B):
3A(g)
↔
2B(g)
The r...

A substance (A) reacts to form another substance (B):
3A(g)
↔
2B(g)
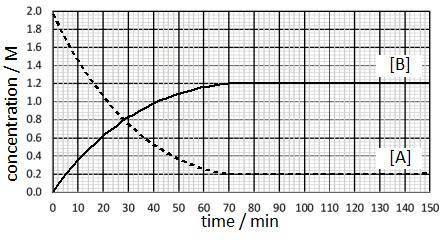
The reaction is run at a particular temperature with the concentrations of A and B monitored over time and plotted in the graph. At what time was equilibrium first reached and what is the approximate value of the equilibrium constant?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
Questions

Arts, 15.12.2020 20:10


Social Studies, 15.12.2020 20:10

Chemistry, 15.12.2020 20:10


Mathematics, 15.12.2020 20:10







Mathematics, 15.12.2020 20:10





Mathematics, 15.12.2020 20:10


Mathematics, 15.12.2020 20:10



