
Chemistry, 08.12.2021 03:00 xlansmooth
3
(a) Represent all products and reactants using VSEPR three dimensional sketches. Include hydrogen atoms. If there are any stereogenic centres present, indicate these with an asterisk (*). If none are present, write a sentence to state this.
(b) Fill in the table on your template to indicate how/if shape and hybridization about C1, C2, C3, and C4 change during this reaction. To do this, sketch, label and populate (with the central atom valence electrons) the hybridized orbitals (and any remaining unhybridized orbitals).

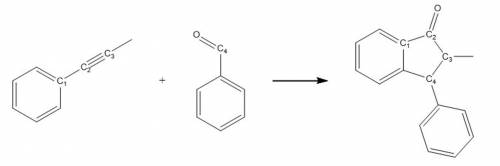

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2


Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

You know the right answer?
3
(a) Represent all products and reactants using VSEPR three dimensional sketches. Include hydroge...
Questions




Mathematics, 21.03.2020 08:55




Mathematics, 21.03.2020 08:56

Biology, 21.03.2020 08:56






Mathematics, 21.03.2020 08:56



Geography, 21.03.2020 08:57


Chemistry, 21.03.2020 08:57



