Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
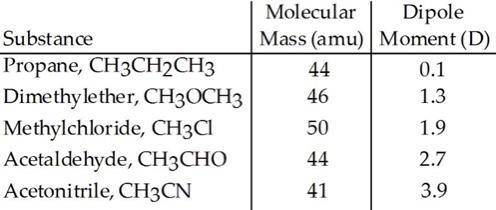
Based on molecular mass and dipole moment of the five compounds in the table below, which should hav...
Questions

History, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31







Mathematics, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31


Mathematics, 19.12.2019 07:31

Mathematics, 19.12.2019 07:31


English, 19.12.2019 07:31




Mathematics, 19.12.2019 07:31