
Chemistry, 06.12.2021 03:40 loganparrish2488
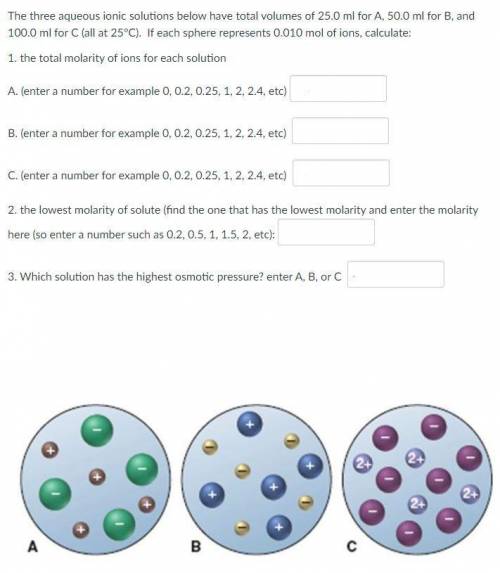
The three aqueous ionic solutions below have total volumes of 25.0 ml for A, 50.0 ml for B, and 100.0 ml for C (all at 25°C). If each sphere represents 0.010 mol of ions, calculate:
1. the total molarity of ions for each solution
2. the lowest molarity of solute
3. Which solution has the highest osmotic pressure?
See the picture attached.
My answers:
1.
A. 3.2
B. 2
C. 1.2
2. 0.4
3. A
Am I right?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1


Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3
You know the right answer?
The three aqueous ionic solutions below have total volumes of 25.0 ml for A, 50.0 ml for B, and 100....
Questions


Mathematics, 24.07.2019 11:30

English, 24.07.2019 11:30


Mathematics, 24.07.2019 11:30

History, 24.07.2019 11:30




Mathematics, 24.07.2019 11:30

Social Studies, 24.07.2019 11:30







Mathematics, 24.07.2019 11:30


Biology, 24.07.2019 11:30



