
Chemistry, 05.12.2021 22:40 azireyathurmond1
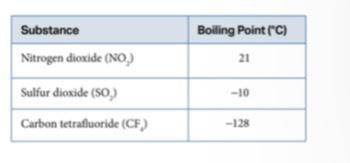
Sulfur dioxide (SO2) and nitrogen dioxide (NO2) both have dipoles, and carbon tetrafluoride (CF4) is nonpolar. All the molecules have relatively similar masses. What could account for the difference in their boiling points? GIVING BRAINLIEST OUT.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1
You know the right answer?
Sulfur dioxide (SO2) and nitrogen dioxide (NO2) both have dipoles, and carbon tetrafluoride (CF4) is...
Questions

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Social Studies, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Chemistry, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Biology, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01

Physics, 16.09.2020 06:01

English, 16.09.2020 06:01

Mathematics, 16.09.2020 06:01



