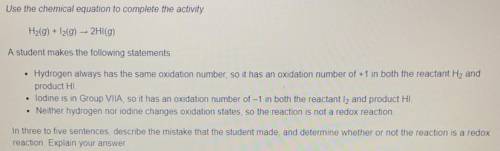
H2(g) + I2(g) -> 2H1(g)

Chemistry, 03.12.2021 06:40 skyhighozzie
Use the chemical equation to complete the
activity.
H2(g) + I2(g) -> 2H1(g)
A student makes the following statements:
- Hydrogen always has the same oxidation number, so it has an oxidation number of +1 in both the reactant H2 and product HI.
- iodine is in Group VIlA, so it has an oxidation number of -1 in both the reactant 12 and product HI.
- Neither hydrogen nor iodine changes oxidation states, so the reaction is not a redox reaction.
In three to five sentences, describe the mistake that the student made, and determine whether or not the reaction is a redox reaction. Explain your answer.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 02:40
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Use the chemical equation to complete the
activity.
H2(g) + I2(g) -> 2H1(g)
H2(g) + I2(g) -> 2H1(g)
Questions

Mathematics, 20.08.2019 19:30

English, 20.08.2019 19:30

Mathematics, 20.08.2019 19:30


Geography, 20.08.2019 19:30

History, 20.08.2019 19:30



Biology, 20.08.2019 19:30


History, 20.08.2019 19:30


Physics, 20.08.2019 19:30

History, 20.08.2019 19:30

History, 20.08.2019 19:30

Business, 20.08.2019 19:30


Mathematics, 20.08.2019 19:30


Biology, 20.08.2019 19:30



