
Select the correct answer.
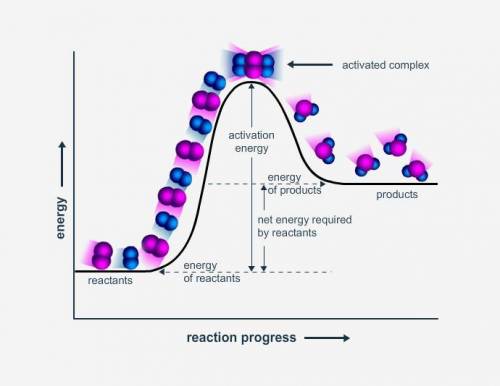
How is the enthalpy of reaction shown in this potential energy diagram?
A.
as the sum of the energy of the products and the energy of the reactants
B.
as the sum of the activation energy and the energy of the products
C.
as the difference of the energy of the reactants and the energy of the products
D.
as the difference of the activation energy and the energy of the products

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
Select the correct answer.
How is the enthalpy of reaction shown in this potential energy diagram?...
Questions



Mathematics, 18.10.2021 07:20


History, 18.10.2021 07:20

Mathematics, 18.10.2021 07:20

Physics, 18.10.2021 07:20


Chemistry, 18.10.2021 07:20

Social Studies, 18.10.2021 07:20



Spanish, 18.10.2021 07:20

English, 18.10.2021 07:20

Chemistry, 18.10.2021 07:20


Computers and Technology, 18.10.2021 07:20

Chemistry, 18.10.2021 07:30

Spanish, 18.10.2021 07:30

Chemistry, 18.10.2021 07:30



