Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 21:30
What is happening when the water inside a kettle heats up and begins to boil
Answers: 1

Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1
You know the right answer?
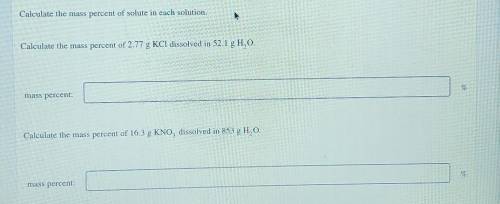
Calculate the mass percent of solute in each solution. Calculate the mass percent of 2.77 g KCl diss...
Questions

Biology, 03.09.2020 22:01

English, 03.09.2020 22:01

Biology, 03.09.2020 22:01


Mathematics, 03.09.2020 22:01

History, 03.09.2020 22:01


Mathematics, 03.09.2020 22:01




English, 03.09.2020 22:01





Mathematics, 03.09.2020 22:01


Mathematics, 03.09.2020 22:01