O CH4

Chemistry, 25.11.2021 14:10 camiilajakobsen1400
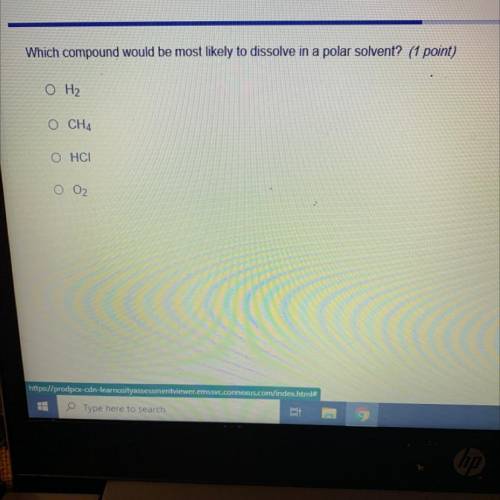
Which compound would be most likely to dissolve in a polar solvent? (1 point)
O H2
O CH4
OHCI
O 02

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1



Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Which compound would be most likely to dissolve in a polar solvent? (1 point)
O H2
O CH4
O CH4
Questions

Computers and Technology, 10.09.2021 14:00

Business, 10.09.2021 14:00

Biology, 10.09.2021 14:00

Biology, 10.09.2021 14:00



English, 10.09.2021 14:10

Biology, 10.09.2021 14:10



Social Studies, 10.09.2021 14:10



Mathematics, 10.09.2021 14:10

History, 10.09.2021 14:10

Mathematics, 10.09.2021 14:10

Mathematics, 10.09.2021 14:10

Mathematics, 10.09.2021 14:10

Chemistry, 10.09.2021 14:10

Mathematics, 10.09.2021 14:10



