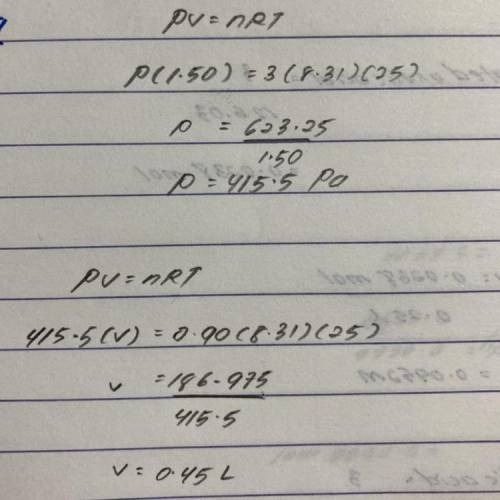Chemistry, 24.11.2021 04:50 camiloriveraveoxbgd6
A balloon is filled to a volume of 1.50 L with 3.00 moles of gas at 25.0 °C. With pressure and temperature held constant, what will be the volume (in L) of the balloon if 0.90 moles of gas are added?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
A balloon is filled to a volume of 1.50 L with 3.00 moles of gas at 25.0 °C. With pressure and tempe...
Questions

History, 10.10.2019 17:30


Mathematics, 10.10.2019 17:30

History, 10.10.2019 17:30

History, 10.10.2019 17:30


Geography, 10.10.2019 17:30

Arts, 10.10.2019 17:30


Chemistry, 10.10.2019 17:30

Mathematics, 10.10.2019 17:30

Mathematics, 10.10.2019 17:30

Physics, 10.10.2019 17:30

Mathematics, 10.10.2019 17:30




Social Studies, 10.10.2019 17:30


History, 10.10.2019 17:30