
Chemistry, 23.11.2021 23:00 runaway173
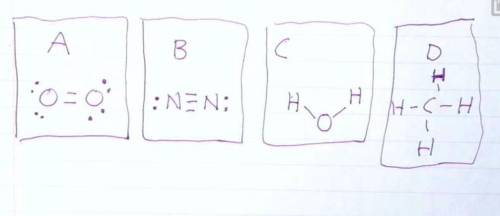
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing on this test and select the answer that best describes which drawing is wrong and why.
Question 6 options:
A: O2 Is wrong because it shows the electrons at a 45 degree angle to the Oxygen atoms.
B: N2 is wrong because it shows a triple bond.
C: H2O is wrong because it is missing 4 valence electrons.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3


Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Draw the Lewis structures of N2, O2, H20, and CH4. Compare your drawing to the ones in the drawing o...
Questions

Mathematics, 10.06.2020 11:57

Mathematics, 10.06.2020 11:57



Mathematics, 10.06.2020 11:57







English, 10.06.2020 11:57

Mathematics, 10.06.2020 11:57


Mathematics, 10.06.2020 11:57


Mathematics, 10.06.2020 12:57





