Chemistry, 22.11.2021 23:50 sadeed00974
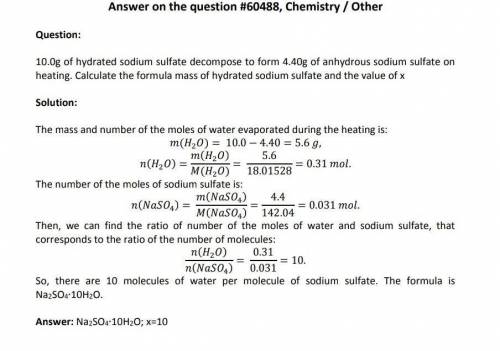
10.00g of hydrated sodium sulfate decomposes to form 4.40g Na(2)SO(4).xH(2)O -> NaSO(4) + xH(2)O of anhydrous sodium sulfate on heating. What’s the formula mass of hydrated sodium sulfate and the value of x? please help i have no clue!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
10.00g of hydrated sodium sulfate decomposes to form 4.40g Na(2)SO(4).xH(2)O -> NaSO(4) + xH(2)O...
Questions



Physics, 05.05.2020 22:58


History, 05.05.2020 22:58



Geography, 05.05.2020 22:58


Mathematics, 05.05.2020 22:58

Arts, 05.05.2020 22:58


Mathematics, 05.05.2020 22:58

Computers and Technology, 05.05.2020 22:58

Mathematics, 05.05.2020 22:58

History, 05.05.2020 22:58