
Chemistry, 10.11.2021 20:10 theatergeek005
Topics: Fundamentals of electrochemistry; Electrochemical cells
Use general chemistry rules for significant figures.
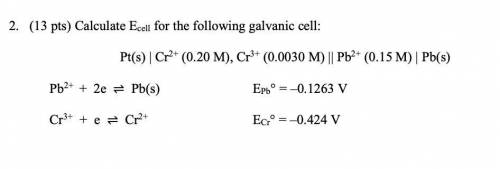
2. (13 pts) Calculate Ecell for the following galvanic cell:
Pt(s) | Cr2+ (0.20 M), Cr3+ (0.0030 M) || Pb2+ (0.15 M) | Pb(s)
Pb2+ + 2e ⇌ Pb(s) EPb° = –0.1263 V
Cr3+ + e ⇌ Cr2+ ECr° = –0.424 V

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1
You know the right answer?
Topics: Fundamentals of electrochemistry; Electrochemical cells
Use general chemistry rules for si...
Questions

Mathematics, 12.04.2021 17:40


Biology, 12.04.2021 17:40

Mathematics, 12.04.2021 17:40

Chemistry, 12.04.2021 17:40

History, 12.04.2021 17:40

Chemistry, 12.04.2021 17:40


French, 12.04.2021 17:40


English, 12.04.2021 17:40

Mathematics, 12.04.2021 17:40


Mathematics, 12.04.2021 17:50

History, 12.04.2021 17:50

Mathematics, 12.04.2021 17:50






