Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:30
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e.g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
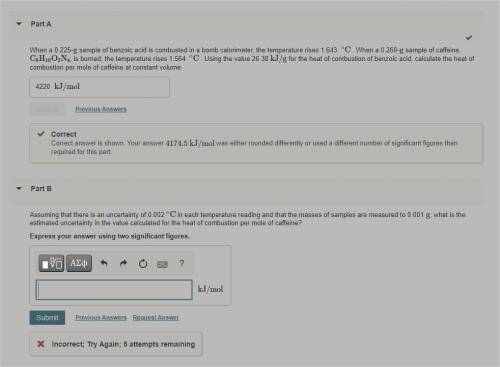
Assuming that there is an uncertainty of 0.002 ∘C in each temperature reading and that the masses of...
Questions

Mathematics, 23.03.2021 22:40




Mathematics, 23.03.2021 22:40

Social Studies, 23.03.2021 22:40

Arts, 23.03.2021 22:40


Mathematics, 23.03.2021 22:40



Mathematics, 23.03.2021 22:40







Mathematics, 23.03.2021 22:40

Business, 23.03.2021 22:40