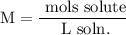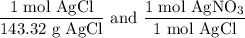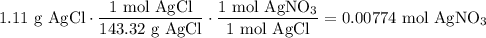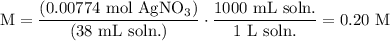Chemistry, 30.10.2021 19:00 jchavez0790
HELPPP PLS IM DESPRATE MAJOR POINTS NEED ASAP
We add excess NaCl solution (58.44 g/mol) to 38 mL of a solution of silver nitrate (AgNO3 169.88 g/mol), to form insoluble solid AgCl. When it has been dried and weighed, the mass of AgCl (143.32 g/mol) is found to be 1.11 grams.
What is the molarity of the original AgNO3 solution? The formula weight of NaNO3 is 85.00 g/mol.
Answer in units of M.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3
You know the right answer?
HELPPP PLS IM DESPRATE MAJOR POINTS NEED ASAP
We add excess NaCl solution (58.44 g/mol) to 38 mL o...
Questions

Chemistry, 27.10.2021 19:00

History, 27.10.2021 19:00

Mathematics, 27.10.2021 19:00

Mathematics, 27.10.2021 19:00



Mathematics, 27.10.2021 19:00

Mathematics, 27.10.2021 19:00

Computers and Technology, 27.10.2021 19:00

Computers and Technology, 27.10.2021 19:00

Mathematics, 27.10.2021 19:00

English, 27.10.2021 19:00




Mathematics, 27.10.2021 19:00

Mathematics, 27.10.2021 19:00


English, 27.10.2021 19:00